About me
I’m a Digital Health Data Scientist. With 9+ years of work experience and solid academic background, my passion lies in exploring innovative digital health applications and biomarker research at the intersection of medicine, AI/ML, statistics, data science, and ubiquitous computing.
I’m currently pursuing a PhD in Applied Machine Learning in the Center for Digital Health Interventions at ETH Zurich.
Before ETH Zurich, I gained extensive expertise in diverse data modalities and analytical techniques in healthcare and medical research as I served as a Senior Data Scientist at Roche specializing in personalized healthcare, a Research Scientist at PRECISIONheor, and a Data Analyst/Biostatistician at Columbia University Medical Center. I obtained a Master’s degree in Biostatistics from Columbia University in the City of New York.
My PhD research focuses on digital biomarkers for aging and healthspan using wearables and digitalizing inflammatory biomarkers for systemic inflammation.
- AI/ML in Healthcare
- Digital Health/Digital Medicine
- Digital Age/Healthspan Biomarker
- Digital Inflammatory Biomarker
- Precision Medicine
- Pharmacoepidemiology
- Multimodal, Real-world Data Analysis
PhD in Applied Machine Learning, 2024
ETH Zurich
MS in Biostatistics, 2012
Columbia University in the City of New York
BS in International Health, 2010
University of Alabama at Birmingham
Experience
PhD Thesis: Towards Digital Biomarker of Aging and Systemic Inflammation Leveraging Machine Learning
Project details can be found in digital biomarkers for longevity using wearables and digitalizing inflammatory biomarkers for systemic inflammation
Research Areas

1.Digital biomarker for longevity & Healthspan
Applying AI/ML and statistical methods for predicting biological age and healthspan using wearable data

2.Explainable AI in biological age estimation
Applying SHAP (SHapley Additive exPlanations) method to identify factors influencing biological age using wearable data

3.Digital phenotyping to demonstrate the impact of circadian aging using wearable data
Enhancing digital phenotyping through the application of AI/ML and statistical models to demonstrate using wearable, time-series data

4.Model-driven and data-driven disease classification
Using AI/ML (CNN, LSTM, RF, DT, etc.) and statistical models (cosinor, SSA) to longitudinal, time-series physical activity data to classify different diseases
5.Digitization of Inflammatory Biomarkers for Systemic Inflammation
Development of digital, multi-modal, and non-invasive inflammatory biomarkers to improve early diagnosis and patient-centered disease monitoring for systemic inflammation
6.Precision medicine using multi-modal, real-world data
Addressing the unmet needs and advancing personalized therapies of cancer patients utilizing multi-modal, real-world data
Featured Work
Selected projects and publications. See all publications here.
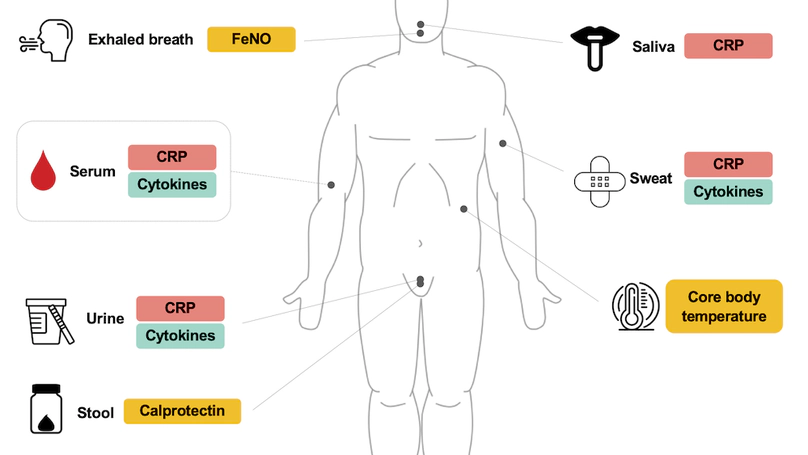
Prolonged systemic inflammation is recognized as a major contributor to the development of various chronic inflammatory diseases. Daily measurements of inflammatory biomarkers can significantly improve disease monitoring of systemic inflammation, thus contributing to reducing the burden on patients and the health care system. There exists, however, no scalable, cost-efficient, and noninvasive biomarker for remote assessment of systemic inflammation. To this end, we propose a novel, multimodal, and noninvasive approach for measuring inflammatory biomarkers. This study aimed to evaluate the relationship between the levels of inflammatory biomarkers in serum (gold standard) and those measured noninvasively in urine, sweat, saliva, exhaled breath, stool, and core body temperature in patients with systemic inflammation. This study is a single-center, cross-sectional study and includes a total of 20 participants (10 patients with systemic inflammation and 10 control patients). Eligible participants provide serum, urine, sweat, saliva, exhaled breath, and stool samples for biomarker analyses. Core body temperature is measured using a sensor. The primary end point is the level of C-reactive protein (CRP). The secondary end points are interleukin (IL)–1β, IL-6, IL-8, IL-10, and tumor necrosis factor-α levels. The tertiary end points are fractional exhaled nitric oxide, calprotectin, and core body temperature. Samples will be collected in 2 batches, enabling preliminary analysis of the first batch (patients 1-5 from each group). The full analysis will include both batches. CRP and cytokine levels will be measured using enzyme-linked immunosorbent assay and electrochemiluminescence immunoassay. For statistical analysis, the Shapiro-Wilk test will be used to evaluate the normality of the distribution in each variable. We will perform the 2-tailed t test or Wilcoxon rank sum test to compare the levels of inflammatory biomarkers between patients with systemic inflammations and control patients. Pearson and Spearman correlation coefficients will assess the relationship between inflammatory biomarkers from noninvasive methods and serum biomarkers. Using all-subset regression analysis, we will determine the combination of noninvasive methods yielding the highest predictive accuracy for serum CRP levels. Participants’ preferences for sampling methods will be assessed through a questionnaire. The study received ethics approval from the independent research ethics committee of Canton Zurich on October 28, 2022. A total of 20 participants participated in the study measurements. Data collection started on February 22, 2023, and was completed on September 22, 2023. Participants were on average 52.8 (SD 14.4; range 24-82) years of age, and 70% (14/20) of them were women. The analysis results reporting findings are expected to be published in 2025. This study aims to evaluate the feasibility of noninvasive, multimodal assessment of inflammatory biomarkers in patients with systemic inflammation. Promising results could lead to the creation of noninvasive and potentially digital biomarkers for systemic inflammation, enabling continuous monitoring and early diagnosis of inflammatory activity in a remote setting.
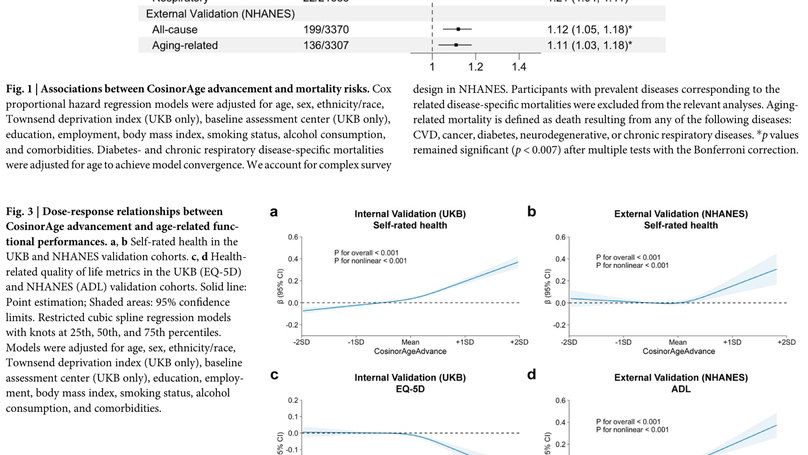
Recognizing the pivotal role of circadian rhythm in the human aging process and its scalability through wearables, we introduce CosinorAge, a novel digital biomarker of aging developed from wearable-derived circadian rhythmicity from 80,000 midlife and older adults in the UK and US. A one-year increase in CosinorAge corresponded to 8-12% higher all-cause and cause-specific mortality risks and 3-14% increased prospective incidences of age-related diseases. CosinorAge also captured a non-linear decline in resilience and physical functioning, evidenced by an 8-33% reduction in self-rated health and a 3-23% decline in health-related quality of life score, adjusting for covariates and multiple testing. The associations were robust in sensitivity analyses and external validation using an independent cohort from a disparate geographical region using a different wearable device. Moreover, we illustrated a heterogeneous impact of circadian parameters associated with biological aging, with young (<45 years) and fast agers experiencing a substantially delayed acrophase with a 25-minute difference in peak timing compared to slow agers, diminishing to a 7-minute difference in older adults (>65 years). Our findings underscore CosinorAge’s potential as a scalable, economic, and digital solution for promoting healthy longevity, elucidating the critical and multifaceted circadian rhythmicity in aging processes. Consequently, our research contributes to advancing preventive measures in digital medicine.
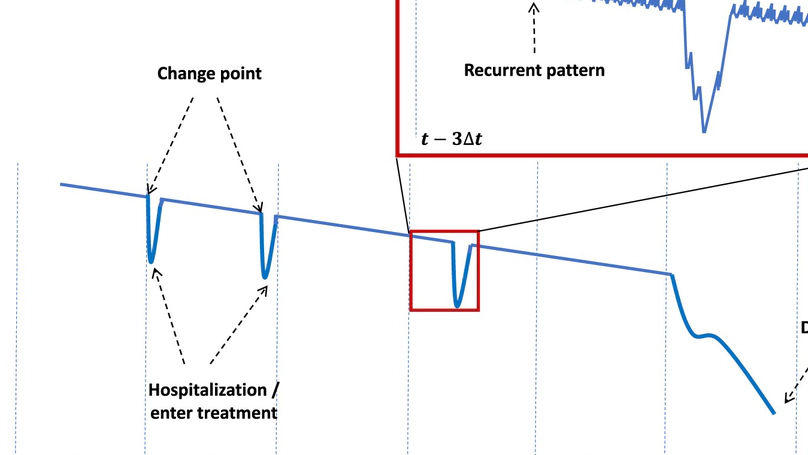
We introduce the Bitemporal Lens Model, a comprehensive methodology for chronic disease prevention using digital biomarkers. The Bitemporal Lens Model integrates the change-point model, focusing on critical disease-specific parameters, and the recurrent-pattern model, emphasizing lifestyle and behavioral patterns, for early risk identification. Results-By incorporating both the change-point and recurrent-pattern models, the Bitemporal Lens Model offers a comprehensive approach to preventive healthcare, enabling a more nuanced understanding of individual health trajectories, demonstrated through its application in cardiovascular disease prevention. We explore the benefits of the Bitemporal Lens Model, highlighting its capacity for personalized risk assessment through the integration of two distinct lenses. We also acknowledge challenges associated with handling intricate data across dual temporal dimensions, maintaining data integrity, and addressing ethical concerns pertaining to privacy and data protection. The Bitemporal Lens Model presents a novel approach to enhancing preventive healthcare effectiveness.
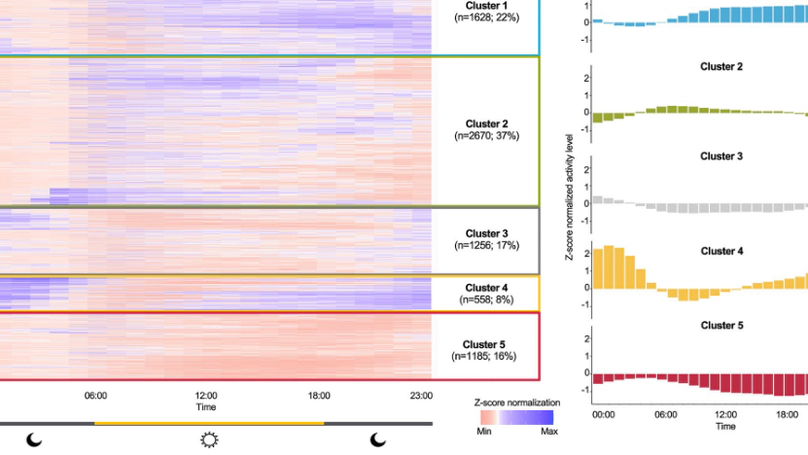
Repeated disruptions in circadian rhythms are associated with implications for health outcomes and longevity. The utilization of wearable devices in quantifying circadian rhythm to elucidate its connection to longevity, through continuously collected data remains largely unstudied. In this work, we investigate a data-driven segmentation of the 24-h accelerometer activity profiles from wearables as a novel digital biomarker for longevity in 7,297 U.S. adults from the 2011–2014 National Health and Nutrition Examination Survey. Using hierarchical clustering, we identified five clusters and described them as follows - “High activity”, “Low activity”, “Mild circadian rhythm (CR) disruption”, “Severe CR disruption”, and “Very low activity”. Young adults with extreme CR disturbance are seemingly healthy with few comorbid conditions, but in fact associated with higher white blood cell, neutrophils, and lymphocyte counts (0.05–0.07 log-unit, all p < 0.05) and accelerated biological aging (1.42 years, p < 0.001). Older adults with CR disruption are significantly associated with increased systemic inflammation indexes (0.09–0.12 log-unit, all p < 0.05), biological aging advance (1.28 years, p = 0.021), and all-cause mortality risk (HR = 1.58, p = 0.042). Our findings highlight the importance of circadian alignment on longevity across all ages and suggest that data from wearable accelerometers can help in identifying at-risk populations and personalize treatments for healthier aging.
Contact
- jshim@ethz.ch
- +41 76 545 7890
- Centre for Digital Health Interventions, ETH Zurich, Weinbergstrasse 56/58, Room G214, Zurich, 8006